Which Group Of Metals Is The Most Reactive
Which Group Of Metals Is The Most Reactive. The attraction from the positive nucleus to the negative electron is less. They must be stored under oil or they will quickly oxidize.
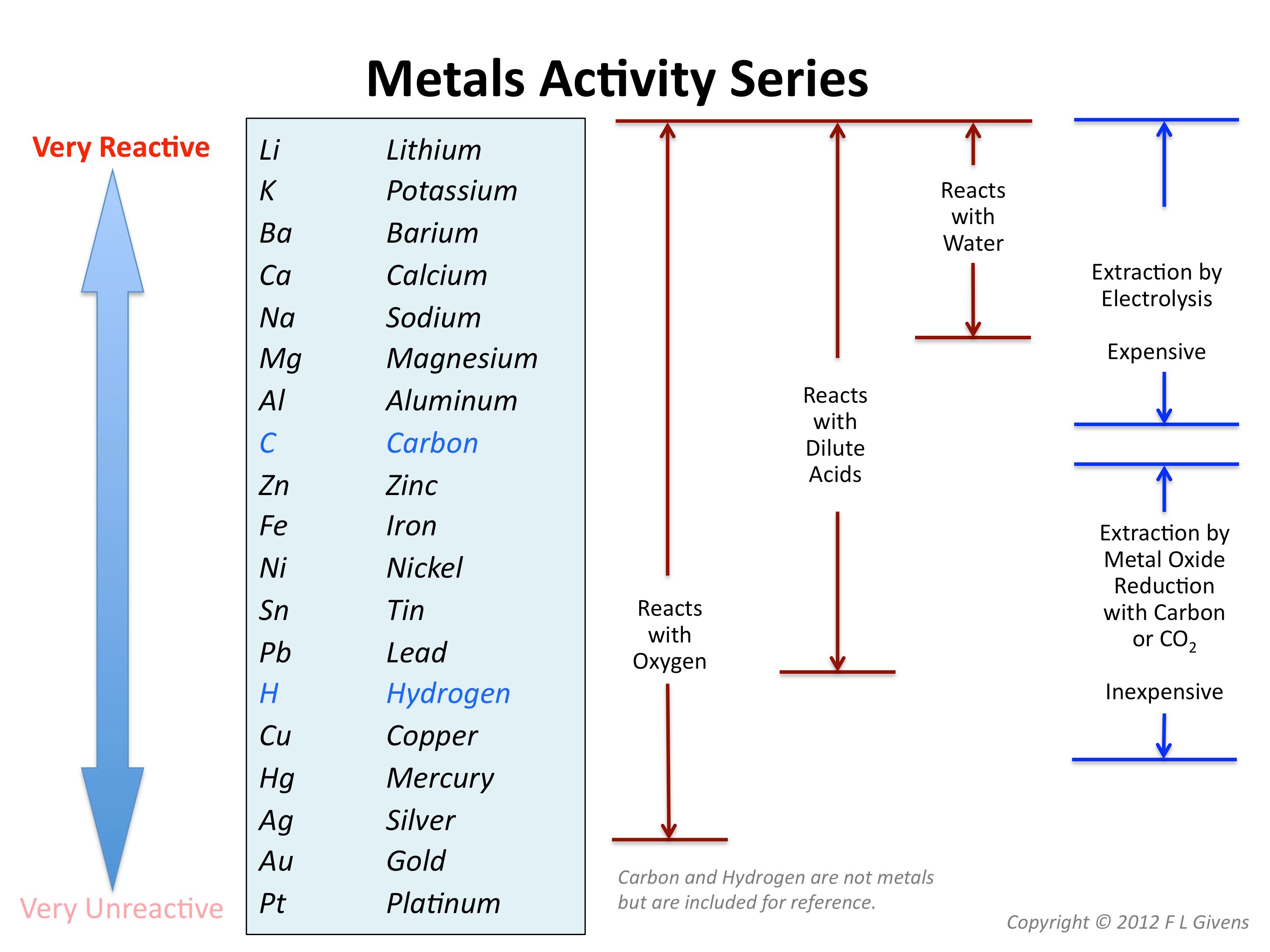
These metals are so reactive that they are not found naturally by themselves; The halogens, alkali metals, and alkaline earth metals are highly reactive. As with all metals, the alkali metals are malleable, ductile, and are good conductors of heat and electricity. The most reactive elementary group is alkali metals (situated far apart from intermediate metals and noble gases). Alkali metals are very reactive and have low melting points.
Similarly, what is the trend of reactivity in group 1?
Group of answer choices a. The halogens, alkali metals, and alkaline earth metals are highly reactive. These metals are characterized by their soft texture and silvery color. Which element in group 2 is most reactive? Therefore, they are ready to lose that one electron in ionic bonding with other elements. They tend to donate their electrons in reactions and have an oxidation state of +1. Group 2 on the periodic table reactive do not occur naturally 2 valence electrons. Since the noble gases are a special group because of their lack of reactivity, the element fluorine is the most reactive nonmetal. Your email address will not be published. They lose this electron very easily, forming ions with a charge of +1. Alkali metals are among the most reactive metals. Alkali metals are very reactive and have low melting points. The least reactive elements are the noble gases.
This same strong reactivity because of one valence electron is true of potassium, as well. The alkali metals consist of the elements lithium, sodium, potassium, rubidium, cesium and francium. The alkali metals, found in group 1 of the periodic table (formerly known as group ia), are very reactive metals that do not occur freely in nature. The most reactive nonmetals reside in the upper right portion of the periodic table. The alkali metals, found in group 1 of the periodic table (formerly known as group ia), are very reactive metals that do not occur freely in nature.
Group of answer choices a.
Therefore, they are ready to lose that one electron in ionic bonding with other elements. Group 1 metals are the most reactive metals on the periodic table and do not exist free in nature. Some examples are potassium, lithium and sodium. Group of answer choices a. The alkali metals consist of the elements lithium, sodium, potassium, rubidium, cesium and francium. The halogens, alkali metals, and alkaline earth metals are highly reactive. The reactivity of alkali metals increases from the top to the bottom of the group, so lithium (li) is the least reactive alkali metal and francium (fr) is the most reactive. Francium is an alkali metal in group 1/ia. Which group of nonmetals is the most reactive? Which group of nonmetals is the most reactive? Leave a reply cancel reply. All the other elements in group 1 are alkali metals. These metals have only one electron in their outer shell.
Leave a reply cancel reply. Plz mark me as brainliest The most reactive group of metals on the periodic table are the alkali metals. The most reactive element is fluorine, the first element in the halogen group. Alkali metals can explode if they are exposed to water.
These metals have only one electron in their outer shell.
Which element in group 2 is most reactive? Beside above, why is francium the most reactive metal? They tend to donate their electrons in reactions and have an oxidation state of +1. Lithium, sodium, and potassium all react with water, for example. Therefore, they are ready to lose that one electron in ionic bonding with other elements. React with hydrogen to form solid hydrides \2m_{(s)} + h_{2(g)} \rightarrow 2mh(s)\ Why is group 2 less reactive? The alkali metals are soft. Group 2 consists of the alkaline earth metals. The reactivity series of metals is a chart listing metals in order of decreasing reactivity. This is due in part to their larger atomic radii and low ionization energies. Alkali metals are very reactive and have low melting points. They tend to donate their electrons in reactions and have an oxidation state of +1.
Komentar
Posting Komentar